It’s 2:30 in the afternoon in the Dominican Republic, and Karen Velline, a 66-year-old grandmother from Cold Spring, Minnesota, is lying on an operating table, swaddled in sterile surgical sheets. She’s just moments away from a procedure so experimental that no doctor will perform it on U.S. soil. Yet she calmly stares up at the ceiling, more excited than anxious. Despite the controversy surrounding it, Velline believes that this procedure—which she has paid Regenocyte Therapeutic, a stem-cell company in Bonita Springs, Florida, $64,000 in cash to perform—could save her from a debilitating lung condition. After months of anticipation and planning, she’s ready for things to get under way.
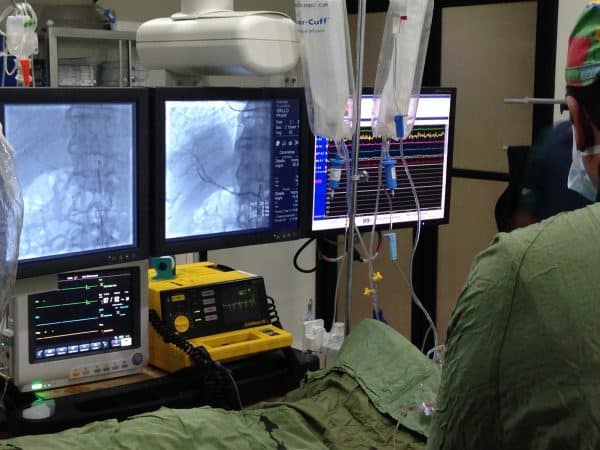
Cardiologist Hector Rosario nods to his team and begins inserting a clear, narrow tube into a vein in Velline’s leg, slowly threading it all the way up to the right side of her heart. “That’s the catheter,” whispers medical supervisor Leonel Liriano, who has agreed to let me watch the surgery. I can see the tube moving on an x-ray imaging screen, inching closer to its final destination, the branched pulmonary arteries that supply blood to her lungs. With the catheter in place, Rosario reaches for a syringe filled with a solution of Velline’s own stem cells: the $64,000 potion. He inserts it into the catheter and depresses the plunger. A subsequent injection of saline serves as a chaser, ensuring that the cells migrate all the way to the lung vessels.
If the technique works as advertised, the cells—hand-couriered on a plane from Israel, where they were mixed with platelet growth factor to make them multiply, and delivered minutes ago to the operating room—will grow into the delicate gas-exchange regions of the lungs. Over several months, they should regenerate failing tissues that have been ravaged by Velline’s hypersensitivity pneumonitis, a degenerative lung disease caused by an allergic reaction to dust and chemicals that has left her dependent on three liters of oxygen a day. Doctors at the Mayo Clinic in Minnesota told her that the only hope of reversing her condition was a lung transplant, a high-risk procedure with a drawn-out recovery period. “That was something I didn’t want to consider,” she says.
Regenocyte presented an enticing alternative. Its glossy brochures and the effusive patient testimonials on its Web site offered hope that stem-cell therapy could not only keep her condition from getting worse but return her to her old self. If regenerative stem cells could help others, Velline reasoned, why couldn’t they help her too? As far as she was concerned, waiting years for the government to put its official seal of approval on the procedure wasn’t an option. She was dying.
Every year, hundreds of desperately ill Americans like Velline are making similar decisions, sidestepping government regulations and heading overseas to access a smorgasbord of stem-cell therapies unavailable in the U.S. Many of these treatments—offered by companies like Regenocyte, Germany’s XCell-Center and China’s Beike Biotechnology—involve autologous adult stem cells, meaning stem cells harvested from your own blood or bone marrow. These are thought to be safer than stem cells drawn from other donors or harvested from embryos, because they incur fewer risks of rejection or tumor formation. Just how safe, though, no one knows precisely, which is why the U.S. Food and Drug Administration insists on stringent regulations.

George Grekos, a Florida-based physician, offers patients experimental stem-cell transplants in the Dominican Republic.
The FDA thinks all stem-cell procedures should undergo clinical trials for safety and efficacy before companies begin selling them as therapies. Its formal review process, the agency maintains, is the only way to protect patients from treatments that are ineffective or downright dangerous.
But with multistage clinical trials lasting up to five years and costing as much as $100 million, a growing number of doctors and patients have started pursuing other options.
Cardiologist George Grekos, Regenocyte’s founder, is at the forefront of this movement. His company is something of a renegade in the stem-cell world: an American firm that offers unsanctioned stem-cell treatments in the Dominican Republic to last-ditch heart and lung patients. Grekos argues that the FDA is unfairly limiting patients’ treatment options, ignoring overseas data and generally overstepping its boundaries. Autologous stem cells, he believes, should fall outside its jurisdiction, just as bone-marrow transplants and in vitro fertilization do.
Back in the operating room, doctors want to inject the rest of Velline’s cells into other vessel groups, but they’ve discovered an air pocket, known as a bulla, in her left lung, forcing them to proceed gingerly. “If they hit the bulla, her lung could explode,” Liriano says. I hold my breath as the tube snakes in and out, probing first one cluster of vessels, then another. Two hours later, the doctors have managed to inject the cells into six different regions of the lungs without incident. As Velline rolls into the recovery room, she is lucid and talking animatedly. “I was awake the whole time,” she says. “I could see everything that was going on. The catheter bobbing up and down in there—it reminded me of a fishing pole.”
Grekos has told Velline up front that there are no guarantees; few patients in the world have ever received autologous-stem-cell therapy for lung ailments. Still, she remains optimistic about her chances for a better future. “I feel so lucky and so blessed,” she says. “I was watching a special on 60 Minutes, and they were talking about stem cells like these treatments were all in the future. I said to myself, ‘Well, I’m getting this now.’ ”
The Bangkok Experiments
George Grekos, MD isn’t your typical white coat. On the day I meet him at his cardiology clinic in Bonita Springs, Florida, he’s wearing jeans, a button-down shirt, and a gold chain around his neck, a casual getup that hints more at his side gig as a restaurant owner than it does at the ruckus he’s creating on the frontiers of regenerative medicine.
A second-generation Greek immigrant who grew up in Ft. Lauderdale, Grekos likes to joke that he started his restaurant business just so he could get good Greek food where he lives. But one gets the sense that he soured on the traditional path of medicine years ago. His 16-year-old daughter, Valentina, has her sights set on medical school, but he’s tried to dissuade her. His disillusionment, he says, stems in part from a malpractice lawsuit filed against him in 1997 after one of his patients died of heart failure. The patient’s family claimed that Grekos should have recommended a coronary bypass sooner, and Grekos’s insurance company wound up settling out of court for $600,000. Grekos felt victimized. “It’s getting harder and harder to be a doctor,” he says. “You have lawyers and government officials trying to dictate to you, when what you’d really like to do is help patients.”

Elizabeth Svoboda
The Shipment
A bag containing a patient’s stem cells is hand-couriered on a plane from Israel to the Dominican Republic.
With stem cells, he saw an opportunity to improve patients’ lives, even if it meant doing an end run around government guidelines. He conceived of the idea for Regenocyte in 2006, when a friend and fellow restaurant owner, Neim Malo, called him for help. Malo was only in his late 40s and had recently suffered a heart attack. Short of a heart transplant, he told Grekos, his doctors in the U.S. were giving him little hope of a full recovery, so he wanted to try something completely unconventional. “He said, ‘I want to go to Thailand, and I want to do stem cells,’ ” Grekos recalls.
After he got off the phone with Malo, Grekos checked out the Web site for the company he had mentioned, TheraVitae, which was headquartered in Toronto and offered stem-cell procedures at Chao Phya Hospital in Bangkok. Technicians draw eight ounces of the person’s blood and courier it to a commercial stem-cell lab in Israel, one of only a handful worldwide that specialize in extracting adult stem cells from the blood and growing them in culture. Once infused into the patient’s body by catheter, the TheraVitae site claimed, the cells turned into new blood vessels, keeping dying muscle tissue alive and encouraging the growth of new cells.
Reading further, Grekos was impressed. In the U.S., autologous-stem-cell treatments other than bone-marrow transplants are only in early-stage clinical trials, and there’s still no definitive proof that they work. Embryonic stem cells, which are thought to be more versatile than adult stem cells because they can give rise to any tissue type in the body, are even less proven. The only embryonic-stem-cell trial announced in the U.S. to date, by the California company Geron, and designed to assess the cells’ therapeutic potential in acute spinal-cord-injury patients, has been repeatedly postponed partly because of regulators’ concerns about the safety of the cells.
TheraVitae, on the other hand, had already published peer-reviewed papers on its scientific methods using autologous adult stem cells in the British Journal of Hematology and the Asian Cardiovascular & Thoracic Annals, among others, testifying to the cells’ robust therapeutic benefit. It had also recently presented early Thai clinical-trial results at an American Heart Association conference. “I’ll go with you for the procedure,” Grekos told Malo. “If anything doesn’t look or smell right, then we’re out of there.”
Grekos’s skepticism evaporated in Bangkok, during a visit to Chao Phya Hospital. Clinical trials there showed cardiac patients thriving after receiving stem-cell injections. In one trial, just six months after the transplant, 21 of 24 patients performing a six-minute walking test were able to stroll 270 feet farther than they could before the transplant. In another trial, involving 40 patients, the therapy improved the heart’s capacity to pump blood by more than 20 percent on average. Then there was his friend’s steadily improving condition. Malo’s pre-treatment ejection fraction—the percentage of blood his heart pumped out with each beat—had been only 30 percent, but six months after treatment, that number had shot up to 50 percent. The recovery was so remarkable, Grekos says, that today Malo’s doctors consider his heart condition cured. Malo says he leads a normal life, working full eight-hour days at the Watermark Grille, the restaurant he still owns in Naples.

Courtesy Regenocyte
The Surgery
Regenocyte cardiologist George Grekos uses a catheter to inject the stem cells into a patient’s heart. The procedure takes about two hours.
Grekos was sold. The cardiologist in him saw an opportunity to help the critically ill, while the seasoned businessman recognized an untapped market—he knew it would probably be many years before patients could obtain similar stem-cell therapies in the U.S. His own treatment program would piggyback on TheraVitae’s science, with patients receiving an initial consultation at his practice in Florida and then flying for treatment to the Dominican Republic, where health authorities support the clinical use of adult stem cells. After training with specialists at TheraVitae, Grekos would perform some of the procedures himself and teach Dominican doctors, such as Rosario, to perform them as well.
With his business plan in hand, Grekos began promoting Regenocyte the same way he might promote a new restaurant: with a full PR campaign. He started touring the country, hosting free seminars that trumpeted the benefits of adult stem cells. Last-chance patients signed up for the procedure in droves. As of early this year, he’s treated 150 people and claims that 80 percent have seen an improvement in their health.
But even as glowing testimonials multiply on Regenocyte’s Web site, scientists in high places have slammed the company’s entire approach to medicine. Neurobiologist and stem-cell innovator Hans Keirstead of the University of California at Irvine calls commercial stem-cell clinics like Regenocyte “deplorable.” “It’s absolutely terrible. They’re taking advantage of people’s need,” he says. And last year, Irving Weissman, head of the International Society for Stem Cell Research (ISSCR), bashed Regenocyte on CNN. “There is no such cell as a regenocyte,” he told a reporter. (Grekos admits as much, explaining that “regenocyte” is not a technical classification but a marketing term his company developed.) “I am disappointed and shocked that somebody would prey on a family that has an untreatable disease,” Weissman said. When presented with the Thai trial in which patients’ ejection fraction improved by approximately 20 percent, Weissman withheld comment on the grounds that the ISSCR has not had a chance to review the paper, but he points out that no regulatory body oversaw the study.
Robert Lanza, the chief scientific officer of U.S. cell-therapy firm Advanced Cell Technology, is equally skeptical of the scientific strength of the Thai trial. “This was a safety—Phase I—trial, so the results don’t really tell us much about the potential efficacy of the cells,” he says.
“There may have been some improvement. However, it’s impossible to reach any conclusion, since the study wasn’t randomized and there was no control group. The study was further compromised by the fact that the cardiologists evaluating the patients were not ‘blinded.’ The slight improvements reported in this paper could easily be the result of a placebo effect.”
Grekos isn’t moved. His detractors aren’t malicious people, he points out; just misinformed. “We’re seeing some amazing clinical results,” he says. In a presentation he gave at a prominent cardiovascular conference in 2008 summarizing the results from an unblinded study of 20 cardiomyopathy patients (with no control group), patients’ ejection fraction improved by an average of 20 percentage points during the six months following the treatment—similar to the results in the Thai trial.

Courtesy Regenocyte
The Spot
Doctors use an x-ray imaging screen to guide the catheter into position.
If, as he claims, his critics are misinformed about his results, it’s partly because Grekos has yet to publish his methodology, even as his business benefits from his open access to peer-reviewed papers on American clinical trials. Grekos says he intends to eventually submit his data for publication and petition the FDA for approval, but for now he’s more focused on treating patients than on writing papers and organizing expensive clinical trials to prove himself to the scientific establishment. “This is what you’ve got to consider,” he says. “What other options do I have? Most of these patients have degenerative diseases, so their prognosis is pretty grim. They’re going to die.”
Stem Cells: Drugs or Body Parts?
Traditionally, the FDA is not in the business of telling doctors how to practice medicine. Its primary domain is medication, which, until recently, has not included tissues derived from your own body. Physicians have long been able to use patients’ own tissues in routine medical procedures—bypass surgeries, for example, or skin grafts—without government oversight. And patients have been receiving their own stem cells in the form of bone-marrow transplants for decades. These types of procedures fall into the “practice of medicine” category and thus have never required FDA approval. Free from those regulations, Grekos says, “orthopedic doctors will extract stem cells, mix them with a bone graft, and use them to heal fractures.”
Now that’s changing. In a controversial move in 2005, the FDA reclassified autologous stem cells that are manipulated by growth factors or other compounds as drugs. This criterion holds whether the cells are derived from a patient’s own body or from someone else’s. Many believe that the policy change gives the agency more authority than Congress ever intended it to have. Grekos’s theory is that pharmaceutical companies are pressuring the FDA to treat autologous stem cells as a drug in order to secure their own future profits. “The drug companies don’t want anything to pass unless they can make money off of it.” Although the FDA says any tissue that’s been manipulated and put back into the body is subject to regulatory oversight, Grekos argues that multiplying the cells and adding growth compound to them is in no way equivalent to turning them into medication. “There’s no genetic manipulation or splicing—you’re not altering the cells at all. All you’re doing is maturing them. The growth factors we use are naturally occurring in the body.”
Yet most scientists and clinicians back the current FDA system on the grounds that requiring investigators to perform double-blind, multistage trials is the only way to protect patients from ineffective or perilous stem-cell therapies.
Without the FDA’s quality-control filter, patients like Velline must decide for themselves which treatments are worth pursuing. And the results, says cardiologist Deepak Srivastava, aren’t always pretty.
“An unbiased body approving these treatments is important so the public has some sort of stamp. I get e-mails frequently from patients around the world saying, ‘I got stem-cell injections, and initially it seemed like it might have helped, but now I’m back in the same boat,’ ” says Srivastava, who directs the Gladstone Institute of Cardiovascular Disease in San Francisco and is a member of the International Society for Stem Cell Research. He stresses that peer-reviewed studies and controlled clinical trials showing objective measures of improvement are the most reliable gauge of a procedure’s promise; video testimonials and glossy patient brochures are just window dressing. But he understands the do-or-die mindset of patients like Velline. “There will be those who are motivated because of the lack of any other options, and I don’t blame them,” he says.

John B. Carnett
In the Saddle
Karen Velline three months after her stem-cell transplant, horseback riding in Arizona. “I already feel like there’s a difference,” she says.
The Hardest Choice
If you or someone you love had a disease that conventional therapies couldn’t cure, would you opt for an experimental therapy like Grekos’s if it represented the only chance—however unproven—of recovery?
So far, Grekos’s customers appear satisfied, and, for now, that seems to be all the validation he needs. Three months after her stem-cell procedure, Velline is recovering with her family in Tucson, Arizona, where she and her husband have a second home. She says she’s suffered very few side effects from the procedure. “A little bit of soreness in my back, but other than that, nothing at all,” she says.
Grekos warned Velline that she might not notice any improvement in her condition for six months or more, but she says she’s already feeling better. “My oxygen at rest is staying higher than it normally does for longer periods of time.” And she’s been jogging on a treadmill every day to build up her strength.
Velline is still awaiting the day when she’ll be able to run, jump, and swim with her grandchildren again, but she is already determined to become an advocate for adult stem-cell procedures, joining Grekos’s ever-swelling ranks of patient-evangelists. “I’m really glad I did it. I feel a little bit of the arrogance here in the U.S., like nobody can do anything right but us.” She hopes to change this attitude by sending digital videos of her procedure to friends with similar health problems, and she’s not particularly concerned about U.S. scientists’ continued criticism of Grekos’s stem-cell procedures. “It doesn’t bother me. I’ve watched people get sick and die from prescription medications, and I’ve talked to people whose lives have been saved by these procedures.”
Elizabeth Svoboda’s most recent feature for Popular Science, in April 2009, was about using viruses unsanctioned by the FDA to treat infections in the U.S.




13 thoughts on “Stem Cell Doctors in the USA Experimental Medicine”
This is the right website for anyone who wants to understand this topic. You understand so much its almost hard to argue with you (not that I really would want toÖHaHa). You certainly put a brand new spin on a subject that has been discussed for many years. Wonderful stuff, just wonderful! This is the right website for anyone who wants to understand this topic. You understand so much its almost hard to argue with you (not that I really would want toÖHaHa). You certainly put a brand new spin on a subject that has been discussed for many years. Wonderful stuff, just wonderful! נערות ליווי במרכז
This is the perfect webpage for everyone who wishes to understand this topic. You realize so much its almost hard to argue with you (not that I personally would want toÖHaHa). You definitely put a brand new spin on a subject that has been written about for a long time. Great stuff, just great!
May I just say what a comfort to uncover somebody who truly knows what theyre talking about on the internet. You actually know how to bring a problem to light and make it important. More and more people should look at this and understand this side of the story. Its surprising you arent more popular because you certainly have the gift.
Im excited to discover this web site. I need to to thank you for ones time for this wonderful read!! I definitely savored every bit of it and I have you bookmarked to look at new information in your web site.
Качественные WordPress ссылки в комментариях от 5000 уник. доменов заказать здесь .
Качественные WordPress ссылки в комментариях от 5000 уник. доменов заказать здесь .
Quality WordPress links in the comments from 5000 uniques. domains order here.
Quality WordPress links in the comments from 5000 uniques. domains order here.
I don’t need to tell you how important it is to optimize every step in your SEO pipeline. But unfortunately, it’s nearly impossible to cut out time or money when it comes to getting good content. At least that’s what I thought until I came across Article Forge… Built by a team of AI researchers from MIT, Carnegie Mellon, Harvard, Article Forge is an artificial intelligence (AI) powered content writer that uses deep learning models to write entire articles about any topic in less than 60 seconds. Their team trained AI models on millions of articles to teach Article Forge how to draw connections between topics so that each article it writes is relevant, interesting and useful. All their hard work means you just enter a few keywords and Article Forge will write a complete article from scratch making sure every thought flows naturally into the next, resulting in readable, high quality, and unique content. Put simply, this is a secret weapon for anyone who needs content. I get how impossible that sounds so you need to see how Article Forge writes a complete article in less than 60 seconds! order here.
I don’t need to tell you how important it is to optimize every step in your SEO pipeline. But unfortunately, it’s nearly impossible to cut out time or money when it comes to getting good content. At least that’s what I thought until I came across Article Forge… Built by a team of AI researchers from MIT, Carnegie Mellon, Harvard, Article Forge is an artificial intelligence (AI) powered content writer that uses deep learning models to write entire articles about any topic in less than 60 seconds. Their team trained AI models on millions of articles to teach Article Forge how to draw connections between topics so that each article it writes is relevant, interesting and useful. All their hard work means you just enter a few keywords and Article Forge will write a complete article from scratch making sure every thought flows naturally into the next, resulting in readable, high quality, and unique content. Put simply, this is a secret weapon for anyone who needs content. I get how impossible that sounds so you need to see how Article Forge writes a complete article in less than 60 seconds! order here.
Use artificial intelligence to cut turnaround time, extend your budget, and create more high-quality content that Google and readers will love. How It Works? WordAi is extremely fast and intuitive. Just enter your content, click rewrite, and in a matter of seconds, WordAi will rewrite an entire piece of content for you. WordAi comes up with different ways to express the same ideas by rewriting every sentence from scratch. This not only removes duplicate content, it also makes the rewritten content read completely naturally. Scale Your Business Make your entire content production process 10x more efficient. Use AI to create more high-quality, unique content that impresses clients and engages readers, all without having to put in more hours Register here and get a bonus.
Use artificial intelligence to cut turnaround time, extend your budget, and create more high-quality content that Google and readers will love. How It Works? WordAi is extremely fast and intuitive. Just enter your content, click rewrite, and in a matter of seconds, WordAi will rewrite an entire piece of content for you. WordAi comes up with different ways to express the same ideas by rewriting every sentence from scratch. This not only removes duplicate content, it also makes the rewritten content read completely naturally. Scale Your Business Make your entire content production process 10x more efficient. Use AI to create more high-quality, unique content that impresses clients and engages readers, all without having to put in more hours Register here and get a bonus.
I was pretty pleased to discover this great site. I need to to thank you for your time for this particularly fantastic read!! I definitely appreciated every part of it and I have you book marked to see new stuff on your blog